Blog
Archive for 2021
Early Detection of Cancer Annual Conference- EDx21 – hosted by Cancer Research UK, OHSU Knight Cancer Institute and the Canary Center at Stanford brought together the brightest minds in cancer early detection research!!
November 29, 2021
 Highlighted excerpts of the conference include:
Highlighted excerpts of the conference include:
• Phil Jones, Ph.D., from the Wellcome Sanger Institute, showed how emerging tumors can be driven to extinction by competing clonal populations of cells.
• Irene Ghobrial, M.D., from Dana-Farber Cancer Institute, also focused on the precursor states, asking whether well-timed interventions can stop the advance to multiple myeloma, an incurable blood cell cancer.
• Minetta Liu, M.D., from Mayo Clinic, gave a whirlwind review of data from several companies readying multicancer, early-detection blood test for broad use, an indicator of the potential for rapid changes in screening practices.
• Mark Emberton, M.D., from University College London, asserted that blood tests will only ever serve as a triage tool for selecting patients for more informative imaging tests.
• Rebecca Fitzgerald, M.D., received the Don Listwin Award for Outstanding Contribution to Cancer Early Detection. She’s known for the development of the Cytosponge, which patients can swallow instead of undergoing an endoscopy.
• Shan Xiang Wang, Ph.D., from Stanford University, highlighted the advantages of giant magnetoresistive (GMR) sensors to look for cancer mutations in circulating DNA, such as EGFR in lung cancer. GMR is more sensitive than fluorescent PCR assays.
• Stephen Friend, M.D., Ph.D., from Oxford University, described studies underway collecting semi-continuous measurements via smartphone, smart ring and smart wristwatch.
• Daniel Heller, Ph.D., at Memorial Sloan Kettering Cancer Center, has developed sensors that can be placed inside the uterine cavity, like an IUD, to detect protein biomarkers of ovarian cancer that take too long to show up in blood.
• Hannah Brewer, Ph.D., from Imperial College London, gave an update on the Cancer Loyalty Card Study, which is tracking changes in medication purchases that could signal early signs of ovarian cancer.
• Aaron Grossberg, M.D., Ph.D., from the OHSU Knight Cancer Institute, asserted that single cells can’t tell the story without the context of what’s going on around them. Organisms have evolved to respond to localized threats in ways that change systemic physiology.
• Peter Kuhn, Ph.D., from the University of Southern California, countered that the single cell holds the necessary information, you just need to find the right cell, the needle in the haystack. It is not necessarily the cell that is a cancer cell, it could be a non-cancer cell that is indicative of the cancer.
• Stacey Fedewa, Ph.D., from the American Cancer Society, contrasted cancer screening among women in the United Kingdom, which provides healthcare to all permanent residents, and the United States, where many go uninsured and cancer screening is opportunistic and not organized with coordinated outreach. But in both countries, only about 4 in 10 women were up to date on cervical, breast and colorectal screenings, and 1 in 10 women in both countries said they received no cancer screening.
• Livia Giordano, M.D., Ph.D., from Centro di Riferimento per l’Epidemiologia e la Prevenzione Oncologica, , made clear the challenges of communication in cancer screening efforts. While it’s important to convey knowledge, inform risk perception to help people make choices that fit their values, Giordano said information is not enough. Communicators must consider emotions and trust.
• Robert Winn, M.D., from VCU Massey Cancer Center, illustrated how in the real world, the introduction of new technologies can create new health disparities, and for too long, medicine has avoided directly addressing the social determinants of health.
• Spencer Robinson, Ph.D., from York and Scarborough Teaching Hospitals NHS Foundation Trust, on a rapid diagnostic service that aims to give a single point of access to diagnosis for all patients who have serious nonspecific symptoms.
• Benjamin Ebert, M.D., Ph.D., from Harvard University, is finding ways to understand the risk of cancer in people with somatic mutations in blood cells, which are extraordinarily common.
• Hans Clevers, M.D., Ph.D., from the Hubrecht Institute, detailed the first patient-derived organoid model for cervical cancer.
• Thea Tlsty, Ph.D., from the University of California San Francisco, detailed efforts using bioengineered culture systems to reveal CAF signaling pathways that could be targeted for therapy or early detection.
• Carolyn Schutt Ibsen, Ph.D., from the OHSU Knight Cancer Institute, has developed a way to simulate, in an in vitro 3D model, the localized oncogene expression found in early-stage cancers.
• Panel discussion – Existing cancer therapies have largely been developed to treat advanced tumors. The panel discussed new therapeutic strategies that will be needed for people diagnosed with very early cancers or pre-cancerous lesions.
• Bissan Al-Lazikani, Ph.D., Institute of Cancer Research/MD Anderson Cancer Center, Early detection faces a signal-to-noise ratio problem that can only be solved by machine and massive, multimodal data mined by AI is the only way we’ll truly unravel the complexity of cancer early detection.
• Xin Lu, FRS F.Med.Sci., with Ludwig Cancer Research and the Oxford Centre for Early Cancer Detection, countered that AI-generated results lack meaning if they can’t be explained by a scientific hypothesis.
The Early Detection of Cancer Conference- EDx21, hosted by: Cancer Research UK, OHSU Knight Cancer Institute & Canary Center at Stanford.
Full daily recap from: Joe Rojas-Burke, Science Writer, OHSU Knight Cancer Institute
Day 1
Henry Scowcroft, a longtime science writer with Cancer Research UK, gained a shattering new perspective on the disease when his partner Zarah was diagnosed with stage IV bladder cancer at age 36. Scowcroft’s recently published memoir of the caregiving experience is interspersed with details of his science-minded effort to understand the disease that took his partner’s life. In the keynote talk, Scowcroft said he was driven to create something that would help others facing cancer, particularly those not privileged with medical knowledge and expert contacts. Caring for Zarah sensitized him to the burden of tests and treatments on cancer patients. He exhorted researchers to always include a focus on making a difference to the lived experience of people with cancer.
When and where detection matters
Cells with cancer-associated mutations become increasingly common with advancing age, but few ever give rise to invasive tumors. Why not? Phil Jones, Ph.D., at the Wellcome Sanger Institute, showed how emerging tumors can be driven to extinction by competing clonal populations of cells. In studies using mouse esophageal epithelium as a model, it was this competition that eliminated incipient tumors, not immune defenses. With deeper understanding, Jones said it could be possible to manipulate this competition of clones to stop cancer.
Irene Ghobrial, M.D., from Dana-Farber Cancer Institute, also focused on the precursor states, asking whether well-timed interventions can stop the advance to multiple myeloma, an incurable blood cell cancer. Ghobrial is principal investigator of the PROMISE Study, the first to screen healthy people at risk for precursor conditions of multiple myeloma, including monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. The study aims to uncover why some patients progress to myeloma and others do not – and inform the development of screening and preventive treatment strategies. In earlier work, her group identified genetic alterations in smoldering multiple myeloma that could distinguish patients at high risk of progression to multiple myeloma.
Breast cancers that emerge in young women within a few years after pregnancy are strikingly more dangerous. Pepper Schedin, Ph.D., with the OHSU Knight Cancer Institute, detailed how tumor cells are shaped by the postpartum environment to become more prone to metastasis. At the end of lactation, 80 to 90 percent of milk-secreting cells undergo programmed cell death, in a process called mammary gland involution. The early phase of gland involution resembles an acute inflammatory response that subsides and is followed by an immune response similar to what happens during wound healing. Schedin said mammary gland involution is an informative model for studying how factors outside of tumor cells drive cancer development.
In the first lightning talk of the conference, Daniel Muñoz-Espin, Ph.D., from the University of Cambridge, explained how targeting senescent cells could be a way to prevent non-small cell lung cancer progression in people presenting with multifocal primary lesions in their lungs. Such lesions pose a treatment conundrum: most will progress to invasive cancer and there is no good way to stop them. Muñoz said accumulation of senescent cells is a common feature. In a mouse model, drug ablation of senescent cells decreased lung tumor burden and increased survival. In some of the mice, the treatment prevented cancer initiation.
Great debate: Blood is the only detection medium that matters
Minetta Liu, M.D., from Mayo Clinic, made the case for blood – an unpopular position going into the debate with the audience poll showing more than 90% disagreeing. Liu pointed out that existing modalities only screen for a few types of cancer. And some, like colonoscopy, are significantly invasive. Staying on schedule with all the separate screening tests is cumbersome for practitioners and, more importantly, burdensome for patients. Blood-based screening, in contrast, involves a point-of-care blood draw to screen for multiple cancers at once. Results so far suggest blood-based tests are pretty good at identifying the tissue of tumor origin to guide follow-up. Liu gave a whirlwind review of data from several companies readying multicancer, early-detection blood test for broad use, an indicator of the potential for rapid changes in screening practices.
Arguing the contrary, Mark Emberton, M.D., from University College London, asserted that blood tests will only ever serve as a triage tool for selecting patients for more informative imaging tests. He said imaging more reliably delivers consistent results while blood test findings can vary considerably from day to day. He said imaging captures a range of attributes – location, volume, morphology, and more – that can’t be derived from a blood test. And imaging, he said, seems to land at a point in tumor development when cancers have the clear potential to become dangerous, while blood tests might detect too many indolent, non-threatening cases invisible on imaging scans. In the end, Liu swayed 19% to her side while support for Emberton’s position dropped to 81%.
Day 2
Rebecca Fitzgerald, M.D., received the Don Listwin Award for Outstanding Contribution to Cancer Early Detection. She’s known for the development of the Cytosponge, which patients can swallow instead of undergoing an endoscopy. Fitzgerald and her team published work showing the device can increase by 10-fold the identification of Barrett’s esophagus, a precursor to esophageal cancer, compared to standard of care. Fitzgerald talked about the power of collaboration, taking risks and the rise of early detection as a hot topic for researchers in an interview with Cancer Research UK.
Presentations kicked off with a session on emerging technologies for detecting and interpreting early signals of cancer. Stanford University’s Shan Xiang Wang, Ph.D., highlighted the advantages of giant magnetoresistive (GMR) sensors to look for cancer mutations in circulating DNA, such as EGFR in lung cancer. GMR is more sensitive than fluorescent PCR assays – and less costly, he said. And GMR can show responses to therapy within two weeks, while CT imaging can take two months or more. Wang’s team is also using GMR to find methylated DNA targets in the blood as a way to detect liver cancer in patients with liver cirrhosis, which produces methylation signatures that are difficult to distinguish from cancer.
Can smartphones and wearables help triage the use of expensive and more invasive tools for cancer early detection? Stephen Friend, M.D., Ph.D., from Oxford University, described studies underway with the nonprofit 4YouandMe. Researchers will collect semi-continuous measurements via smartphone, smart ring and smart wristwatch. They will retrospectively analyze relative changes of multi-modal data streams among those who did and did not demonstrate new tumor growth.
Most ovarian cancers are detected dangerously late. Daniel Heller, Ph.D., at Memorial Sloan Kettering Cancer Center, has developed sensors that can be placed inside the uterine cavity, like an IUD, to detect protein biomarkers of ovarian cancer that take too long to show up in blood. In mouse models of ovarian cancer, the carbon nanotube based optical sensors were stable for at least a month after implantation and reliably detected HE4 protein.
In lightning talk, Hannah Brewer, Ph.D., from Imperial College London, gave an update on the Cancer Loyalty Card Study, which is tracking changes in medication purchases that could signal early signs of ovarian cancer. Her team has recruited 117 women diagnosed with ovarian cancer (cases) and 420 women without ovarian cancer (controls). Comparison of loyalty card data of cases and controls has revealed an increase in purchases of pain and indigestion medications prior to diagnosis, suggesting it might be feasible to use such data to prompt medical screening.
Great Debate: Emergent properties of the ecosystem are how we’ll understand cancer; a focus on a single cancer cell’s biology is misguided
Single cells can’t tell the story without the context of what’s going on around them, asserted
Aaron Grossberg, M.D., Ph.D., from the OHSU Knight Cancer Institute. Organisms have evolved to respond to localized threats in ways that change systemic physiology, he said. Given that the probability of a positive finding in cancer screening is every low, even if cancer is present, he said that bio-amplification of the signal may be an essential process for detecting cancer. And knowing the prevalence of cancer-associated mutations in non-cancerous tissues, the state of single cells may be misleading without also knowing the state of the microenvironment.
Peter Kuhn, Ph.D., from the University of Southern California, countered that the single cell holds the necessary information, you just need to find the right cell, the needle in the haystack. It is not necessarily the cell that is a cancer cell, it could be a non-cancer cell that is indicative of the cancer, he added. Kuhn changed a few minds; before the debate 75% agreed with Grossberg and 25% disagreed. After, 72% agreed and 28% disagreed.
Early detection in the real world
Starting the day’s final session, Stacey Fedewa, Ph.D., from the American Cancer Society, contrasted cancer screening among women in the United Kingdom, which provides healthcare to all permanent residents, and the United States, where many go uninsured and cancer screening is opportunistic and not organized with coordinated outreach. But in both countries, only about 4 in 10 women were up to date on cervical, breast and colorectal screenings, and 1 in 10 women in both countries said they received no cancer screening.
Livia Giordano, M.D., Ph.D., from Centro di Riferimento per l”Epidemiologia e la Prevenzione Oncologica, made clear the challenges of communication in cancer screening efforts. It is essential to maximize informed choice to participate but also informed choice not to participate in screening, just as it is essential to minimize disinformed participation in addition to minimizing involuntary non-participation. While it’s important to convey knowledge, inform risk perception to help people make choices that fit their values, Giordano said information is not enough. Communicators must consider emotions and trust.
In the real world, the introduction of new technologies can create new health disparities, pointed out Robert Winn, M.D., of VCU Massey Cancer Center. He traced the history of Richmond, Virginia, to illustrate how generations of societal policies have marginalized populations to create health disparities, from segregationist real estate practices, to the concentration of polluting industries in poor neighborhoods. For too long, he said, medicine has avoided directly addressing the social determinants of health.
The day concluded with a lightning talk on a rapid diagnostic service presented by Spencer Robinson, Ph.D., from York and Scarborough Teaching Hospitals NHS Foundation Trust. The service aims to give a single point of access to diagnosis for all patients who have serious nonspecific symptoms. General practitioners refer patients. A triage clinician determines those who will receive a default workup including a CT scan and a trans-nasal endoscopy. Robinson said 336 referrals have resulted in 27 cancer diagnoses, including, in order of prevalence, metastases of unknown origin, pancreatic tumors, gynecologic cancers and lymphomas.
Day 3
Our final day started with a session on models and systems to inform detection. Harvard University’s Benjamin Ebert, M.D., Ph.D., is finding ways to understand the risk of cancer in people with somatic mutations in blood cells, which are extraordinarily common. Access to hundreds of thousands of exomes from peripheral blood is making it possible to trace the path from clonal hematopoesis of indeterminate potential to life-threatening cancer, and to identify events that signal escalating risk. Abnormal blood count is one such event.
Hans Clevers, M.D., Ph.D., at the Hubrecht Institute, detailed the first patient-derived organoid model for cervical cancer, developed in his lab by Kadi Lohmussaar, Ph.D. Ordinary Pap sampling provides enough starting material to grow either healthy organoids or cervical cancer tumoroids. The organoids reproduce gene expression patterns of the organ, as the tumoroids mimic the mutations, gene expression and histology of patient tumors.
Cancer associated fibroblasts, or CAFs, play a role in malignant initiation and progression. Thea Tlsty, Ph.D., from the University of California San Francisco, detailed efforts using bioengineered culture systems to reveal CAF signaling pathways that could be targeted for therapy or early detection. In an air-liquid interface culture system, bronchial epithelial cells grown alongside CAFs acquire squamous characteristics. Adding stress signaling triggers high-grade neoplasia. The next step will be to reproduce the development of full-on cancer.
The session closed with a lighting talk by Carolyn Schutt Ibsen, Ph.D., from the OHSU Knight Cancer Institute. Her team has developed a way to simulate, in an in vitro 3D model, the localized oncogene expression found in early-stage cancers. The system uses lipid-monolayer gas-core microbubbles containing plasmid DNA. Directed ultrasound releases the plasmid DNA from the microbubbles so that only cells in ultrasound focal zone are transfected.
What do we do once we detect early?
Existing cancer therapies have largely been developed to treat advanced tumors. This panel of experts discussed the new therapeutic strategies that will be needed for people diagnosed with very early cancers or pre-cancerous lesions. Chair Charles Swanton, Ph.D., from the Francis Crick Institute/UCL, highlighted a daunting challenge: advances in early detection are revealing that some early-stage cancers can be as deadly as later stage disease. Tom Beer, M.D., at the OHSU Knight Cancer Institute, noted encouraging data suggesting that multi-cancer early detection blood tests might be better at detecting aggressive disease than indolent. Susan Galbraith, M.D., Ph.D., from AstraZeneca, highlighted the need for well tolerated therapies for patients diagnosed with early-stage disease. Avi Spira, M.D., with Johnson & Johnson said localized delivery of drugs could be one way to minimize toxicity. Usha Menon, M.D., University College London, expressed hope for linking screening trials with treatment trials; subjects found to have early disease could be randomized to trials of various treatments. All agreed that understanding the biology of early stage and pre-malignant disease will be critical to finding therapeutic targets for treating early cancers and intercepting pre-malignant disease with less toxic treatments.
Great debate: It’s time to give up on human hypothesis-driven research; massive, multimodal data mined by AI is the only way we’ll truly unravel the complexity of cancer early detection
Relying on hypothesis-driven research is like wandering through an infinite jungle hoping to stumble on the correct path, asserted Bissan Al-Lazikani, Ph.D., Institute of Cancer Research/MD Anderson Cancer Center. Early detection faces a signal-to-noise ratio problem, she said, that can only be solved by machine. She pointed to the development of BRAF inhibitors. BRAF emerged as a worthwhile target from computational analysis of massive amounts of DNA sequencing data, not from a scientist’s hypothesis about that particular signaling pathway.
Xin Lu, FRS F.Med.Sci., with Ludwig Cancer Research and the Oxford Centre for Early Cancer Detection, countered that AI-generated results lack meaning if they can’t be explained by a scientific hypothesis. A machine learning algorithm may find complex patterns linked to early cancer, but without a hypothesis to explain them, it’s impossible to validate the truth, Lu said. And AI requires a starting point and training data to learn from, which Lu said requires a human-generated hypothesis about the way the world works.
Going in, 82% of the audience sided with Lu, and 18% with Al-Lazikani. After the debate, Lu’s support dropped to 54% and 46% agreed with Al-Lazikani.
It’s been a productive three days!
On behalf of Cancer Research UK, the OHSU Knight Cancer Institute and the Canary Center at Stanford, thank you for joining us for the 2021 Early Detection of Cancer Conference.
Farewell,
The Early Detection of Cancer Conference events team
Canary Scientists are on the right track!
October 14, 2021
Canary Center continues as a world class facility, acting as a hub for innovative research, collaborations, cross-disciplined studies, and international partnerships.
After their train ride, our scientists gave an informative update at a recent meeting observing distancing measures. Subjects covered included the Canary Center at Stanford, Ovarian and Prostate Programs and examples of work from the lab of Dr. Joseph DeSimone, who has been appointed as the inaugural Sanjiv Sam Gambhir Professor in Translational Medicine.
Below are two highlights and a link to a 2-page report.
Canary Ovarian Initiative is focusing on the microenvironment of the fallopian tubes, high grade serous carcinoma originates in the fallopian tubes, to look for changes that signal cancer.
- Using bioinformatics and methylated DNA to determine origins of ovarian cancer.
- Looking for changes in the fallopian tubes decades before cancer can be diagnosed, especially for high risk women (i.e. BRCA mutations). Single cell sequencing – looking for changes in cells that can signal cancer early.
- Remaining patient-focused for compassion and team-focused for efficiency.
Prostate Cancer Team and the Prostate Active Surveillance Study (PASS), more than a decade on, helps patients in the study as well as informing the medical profession on ways to understand which men are at greatest risk, requiring aggressive treatment versus those who have slow growing cancer. Recent Pass accomplishments include:
- Advanced imaging with MRI.
- African Americans do not have worse outcomes in active surveillance
- Created models to predict non-progression.
- Fewer painful prostate biopsies.
- PASS Risk calculator to aid patients and physicians with decisions.
- High risk patients (BRCA mutation) personalized screening.
Read the full update report here:
Don Listwin Award For Outstanding Contribution to Cancer Early Detection 2021 goes to Rebecca Fitzgerald, MD, FMedSci
October 11, 2021

The Don Listwin Award for Outstanding Contribution to Cancer Early Detection recognizes a sustained contribution to, or singular achievement in, the cancer early detection field.
The 2021 Award goes to: Rebecca Fitzgerald MD FMedSci, MRC Cancer Unit, University of Cambridge and an internationally recognized pioneer for her exceptional research into the prevention and detection of oesophageal cancers.
This award was announced at the recent Early Detection of Cancer Conference – EDx21.
This award is given to recognize and thank Rebecca for the work she has done to develop, grow and establish the research needed to detect cancer early.
She is the Interim Director of the MRC Cancer Unit, Hutchison-MRC Research Centre, Professor of Cancer Prevention, and Clinician Scientist leading research in the Early Detection of Cancer for the University of Cambridge and the CRUK Alliance for Cancer Early Detection (ACED). Rebecca is known for the development of the Cytosponge technology, a sponge on a string that patients can swallow instead of undergoing an endoscopy. The Cytosponge collects cells from the oesophagus for staining, which can flag the presence of TFF3-positive cells indicative of Barrett’s oesophagus, a precursor to oesophageal cancer. Recently Rebecca and her team published work demonstrating that Cytosponge increases the identification of Barrett’s in individuals with frequent heart-burn symptoms by 10-fold compared to standard of care. The building of evidence for its clinical implementation for surveillance of high-risk individuals and in endoscopy sparing due to COVID-19 related pressures on health systems continues to make a vital impact to patients’ lives and is internationally recognized for its contribution towards breaking barriers in research.
Congratulations to Rebecca and we are pleased to have her within the early detection community.
The 2021 Gambhir Symposium (virtual) –celebrating ongoing work of visionary and pioneer Dr. Sanjiv Sam Gambhir
August 6, 2021

The 2021 Gambhir Symposium (virtual) held on July 19, 2021 included discussions and presentations celebrating the continuing work on paths forged by Sanjiv Sam Gambhir, MD, PhD, (1962-2020) in the fields in which he launched new directions:
Molecular Imaging, Cancer Early Detection, Precision Health.
Click Here to see the Conference Agenda & Videos.
Click here to see the videos from the Cancer Early Detection Session.
2021 Virtual PHIND Symposium
April 13, 2021

Dr. DeSimone gestures wearing an Oura monitoring ring, which made the news recently as the company partnered with the National Basketball Association (NBA) to help monitor the health of players.
The 2021 Precision Health and Integrated Diagnostics (PHIND) Center at Stanford held the virtual PHIND Symposium on March 23, 2021. The event showcased the exciting PHIND work that is going on campus-wide, featuring current PHIND investigators and Precision Health experts. Professor of Radiology Dr. Garry Gold gave an update on the PHIND center, reflecting upon former Radiology Chair Dr. Sanjiv Sam Gambhir’s visionary leadership and the continuation of this work, now with Dr. Gold’s direction, aimed at monitoring health to identify early transitions from health to disease.
Discussions included one with Dr. Joseph DeSimone, recently named recipient of the Sanjiv Sam Gambhir Professorship in Translational Medicine, who stated that although he is new to PHiND, his group has been thinking about precision delivery, using new devices such as microneedles to deliver treatments locally, rather than systemically throughout the body. Dr. Utkan Demirci, leading the Canary Center at Stanford, discussed his group’s Exosome-Total-Isolation-Chip (ExoTIC) device for identification of exosome-based biomarkers for monitoring health from a variety of biological fluids.
Besides devices, like the one in the picture, there was a focus on data. What kinds of data are we capturing as the clinical enterprise has transformed through Covid and telehealth? Can we separate patients into cohorts with online and in person visits to optimize the clinical workflows? How can we access and mine data from non-identifiable electronic health records? Participants also discussed ways to ensure equity in access to these devices and data, as well as ways to ensure clinical trial participants are engaged in meaningful ways in monitoring their health.
The Precision Health and Integrated Diagnostics Center (PHIND) at Stanford is dedicated to longitudinal monitoring and improvement of overall human health on a lifelong basis. Stanford advancements in biology and technology are leading to the potential to understand disease risk, detect disease early and enable preventative interventions.
Click Here to watch the videos from the Symposium.
Canary Foundation’s Ovarian Cancer Initiative: moving forward with matching specimen and imaging tissue in 3D
March 8, 2021

The Canary High Grade Serous Ovarian Cancer (HGSC) study is leveraging the expertise and resources of four institutions to study the microenvironmental factors that can lead fallopian tubes to develop this deadly type of ovarian cancer and thus provide a signal to alert for the presence of early disease. The Fred Hutchinson Cancer Research Center in Seattle, the University of Pennsylvania, the Van Andel Institute in Michigan, and the University of California San Francisco have built the infrastructure to share fallopian tube specimens, experimental and clinical data, and analytical teams.
The small pilot study goals are to ask whether it is possible to compare women carrying the BRCA mutations (who are at higher risk of developing ovarian cancer) compared to those who do not carry the mutation and determine whether it is possible to find a measurable difference in the microenvironment.
Based on their first results, the team is selecting a larger set of specimens, matched for clinical factors and BRCA mutation status, and will conduct RNA, DNA and methylome sequencing. The group is also comparing competing platforms for imaging the tissue expression in 3D so that differences along the length of the tube can be evaluated and tested for correlations with the genetic data.
The Canary Center is utilizing expertise to enhance COVID-19 vaccinations
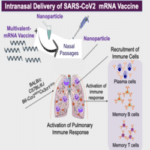
COVID-19 is caused by a novel strain of coronavirus called SARS-CoV-2, which infects cells along the respiratory tract. Early on in the pandemic, Dr. Ramasamy Paulmurugan’s research group sought to explore a non-invasive and efficient vaccination strategy to raise the immune system to fight against COVID-19 at the initial site of infection: the nasopharyngeal region and the lungs.
The pulmonary immune system is considered a major immune organ in humans, containing the majority of T cells, which respond rapidly to infections in the respiratory tract. Hence, developing a vaccination approach for respiratory mucosal immune system can mount a strong immune response against the virus and raise the amount of resident memory B and T cells to provide a long-lasting neutralizing and cell mediated immunity to infectious agents.
With this promising scope in sight, Dr. Paul’s research group is exploring intranasal delivery of a multivalent mRNA vaccine, that effectively induced antibodies as early as two weeks after two doses of the DNA vaccine. This is expected to provide a long-term immunity to COVID-19.
Currently the team is expanding the study to multivalent mRNA vaccine for its wide spectrum antibody to encounter a range of mutant strains, which are dominating and contributing to second and third waves of infection around the world.
Canary’s International Collaboration – ACED

The International Alliance for Cancer Early Detection (ACED) joins researchers from the United States and the United Kingdom in a $70 million partnership. Founded in 2019, ACED is a partnership with the Canary Center at Stanford University, CRUK, the University of Cambridge, the Knight Cancer Institute at Oregon Health and Science University (OHSU), University College London and the University of Manchester. The following is one study chosen for it’s innovative approach to early detection:
Stratifying Risk for Early Detection in Hereditary Breast and Ovarian cancer
Project Award, led by: Marc Tischkowitz, University of Cambridge; Allison Kurian, Canary Center at Stanford for Cancer Early Detection; and Gareth Evans, University of Manchester. Stanford Team: Allison Kurian, Alice Fan, James Ford
CanRisk is a cancer risk assessment tool which combines genetic, lifestyle, clinical and imaging data to calculate an individual risk estimate for women with high-risk mutations in BRCA1 and BRCA2. The ability to provide personalized cancer risk estimates will identify women at particularly high risk. Currently, the ranges of cancer risk estimates for women with hereditary mutations in breast cancer genes are wide and not personalized, so all women are given the same figures. Creating a customized approach can solve this problem.
By implementing personalized risk estimates, early detection strategies can be tailored for the individual, therefore identifying those at the highest risk. Once feasibility is assessed, women undergoing predictive testing for BRCA1, BRCA2, PALB2, ATM or CHEK2 in US and UK genetics centers will be randomized to conventional vs personalized risk estimate based on genetic/lifestyle/hormonal modifiers.

 Highlighted excerpts of the conference include:
Highlighted excerpts of the conference include: